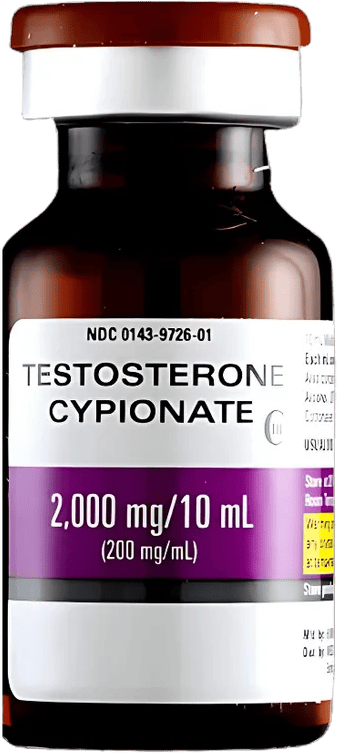
Testosterone Cypionate
Testosterone Cypionate Injection is a compounded preparation of testosterone cypionate suspended in grapeseed oil for deep intramuscular administration. It is available through our 503A and 503B pharmacy operations and is prepared for individual patients pursuant to a valid prescription from a licensed healthcare provider. As a compounded preparation, it is not an FDA-approved drug product, and FDA does not review compounded medications for safety, efficacy, or quality.
Important: Testosterone is classified as a Schedule III controlled substance under the Controlled Substances Act. It may only be dispensed pursuant to a valid prescription and is subject to state and federal monitoring requirements designed to prevent diversion and misuse.
Overview
Testosterone Cypionate Formulations
| Vial Size | Concentrations Available | Carrier Oil |
|---|---|---|
| 5 mL | 20 / 50 / 100 / 200 mg/mL | Grapeseed oil |
| 2.5 mL | 200 mg/mL | Grapeseed oil |
| 10 mL | 200 mg/mL | Grapeseed oil |
| 30 mL | 200 mg/mL | Grapeseed oil |
Multiple concentration options allow prescribers to match the formulation precisely to each patient's dosing requirements and injection volume preferences. Patients with known sensitivities to grapeseed oil or other components should inform their prescriber, as the carrier oil may be adjusted to accommodate individual needs.
What Is Testosterone Cypionate?
Testosterone cypionate is a long-acting esterified form of testosterone — the primary male sex hormone and the principal androgen produced by the testes. The cypionate ester is a fatty acid chain attached to the testosterone molecule that slows its release from the oil depot formed at the injection site. Following intramuscular injection, the ester is gradually cleaved by tissue esterases, releasing free testosterone into systemic circulation over a period of approximately one to two weeks. This extended release profile allows for less frequent dosing compared to shorter-acting testosterone preparations.
Once released, free testosterone circulates through the bloodstream — roughly 98% bound to carrier proteins (sex hormone-binding globulin and albumin) with the remaining 2% available as biologically active free testosterone. Free testosterone enters target cells and binds to androgen receptors in the cytoplasm. The activated receptor complex then translocates to the cell nucleus, where it directly regulates the transcription of genes involved in protein synthesis, red blood cell production, bone mineralization, fat distribution, sexual function, and mood.
Within target tissues, a portion of testosterone undergoes further conversion. In skin, hair follicles, and the prostate, the enzyme 5-alpha reductase converts testosterone to dihydrotestosterone (DHT), a more potent androgen that drives many secondary sexual characteristics. In adipose tissue, the enzyme aromatase converts a fraction of testosterone to estradiol, which plays a role in bone health, libido, and the negative feedback loop that regulates gonadotropin secretion from the pituitary gland. Both conversion pathways are clinically relevant — DHT excess is associated with prostate growth and hair loss, while estradiol imbalance can cause gynecomastia and mood disturbances — and both should be monitored during long-term testosterone therapy.
Simple. Medical. Personalized.
Medical intake
Answer a few online questions about your health history, lifestyle, and goals — no clinic visits required.
Provider evaluation
A licensed medical professional reviews your intake and determines the safest, most effective option for you.

Personalized plan
Custom treatment plan built around your unique health needs, goals, medical history, and biology.

Ongoing support
Stay connected with your care team for follow-ups, adjustments, and expert answers — all online.
FAQs
Clinical Indications
Testosterone Cypionate Injection is prescribed for adult males with confirmed hypogonadism — a clinical condition characterized by the failure of the testes to produce adequate testosterone, resulting in both symptomatic and biochemical deficiency. Hypogonadism may be primary (originating in the testes) or secondary (resulting from insufficient pituitary or hypothalamic stimulation), and both forms may be appropriate indications for testosterone replacement therapy.
Diagnosis requires more than a single low testosterone reading. Current clinical guidelines generally require two morning serum testosterone measurements on separate days that fall below the established lower limit of the normal adult reference range, combined with symptoms consistent with deficiency. Symptoms may include reduced libido, erectile dysfunction, decreased energy, loss of lean body mass, increased fat mass, mood changes including depression or irritability, difficulty concentrating, and reduced bone density.
Compounded testosterone cypionate may also be appropriate for patients who have documented allergies or sensitivities to the inactive ingredients in commercially available testosterone products, or who require a concentration or vial size not offered by manufactured preparations.
Dosage & Administration
Dosage: Determined exclusively by the prescribing healthcare provider based on baseline serum testosterone levels, symptom severity, body weight, and individual treatment goals. Do not self-adjust your dose or injection frequency.
Typical initiation range: 100–200 mg administered intramuscularly every two weeks is a common starting point, though many clinicians prefer more frequent lower-dose injections (e.g., 50–100 mg weekly) to reduce peak-to-trough fluctuations in serum testosterone and minimize associated side effects. Dose and frequency are individualized and adjusted based on follow-up lab work.
Route: Deep intramuscular (IM) injection into the gluteal muscle is standard. Some practitioners use subcutaneous administration for smaller volumes — discuss the appropriate route with your prescriber.
Needle guidance: A 22-gauge needle of appropriate length for the injection site and patient body composition is typically used for IM administration. The injection site should be rotated with each dose.
Injection technique: Aseptic technique is essential. Clean the injection site with an alcohol swab and allow it to dry fully before injecting. Inject slowly and steadily. Dispose of used needles and syringes in an approved sharps container immediately after use — never recap or reuse needles.
Missed doses: If a dose is missed, administer it as soon as possible unless the next scheduled dose is imminent. Never double a dose. Contact your provider for guidance if multiple doses have been missed.
Laboratory Monitoring
Testosterone replacement is not a set-and-forget therapy. Regular laboratory monitoring is a clinical requirement, not optional. At minimum, patients should expect baseline testing before initiation and periodic follow-up assessments of serum testosterone (trough level, drawn just before the next scheduled injection), hematocrit and hemoglobin, prostate-specific antigen (PSA), and a lipid panel. Liver enzymes may also be monitored depending on individual clinical history.
Hematocrit requires particular attention. Testosterone stimulates erythropoiesis (red blood cell production), which can cause hematocrit to rise. If hematocrit exceeds 54%, dose reduction, increased injection frequency to flatten the peak, or therapeutic phlebotomy may be necessary to reduce thromboembolic risk.
PSA should be measured at baseline and at regular intervals thereafter, as testosterone therapy can stimulate prostate tissue growth and may unmask subclinical prostate pathology. This does not mean testosterone causes prostate cancer, but it does mean men with existing or suspected prostate disease require careful evaluation before and during therapy.
Contraindications
Testosterone Cypionate Injection is contraindicated in the following circumstances and must not be initiated or continued in these patients:
Prostate or breast carcinoma: Testosterone is absolutely contraindicated in men with known or suspected carcinoma of the prostate or breast. Androgen-sensitive tumors can be stimulated by exogenous testosterone.
Hypersensitivity: Contraindicated in patients with known hypersensitivity to testosterone, grapeseed oil, or any other component of the formulation.
Pregnancy: Testosterone is teratogenic. It is absolutely contraindicated in women who are pregnant or may become pregnant. Prenatal exposure to androgens can cause virilization of a female fetus. This medication is not intended for use in women in most clinical contexts and is never appropriate during pregnancy.
Breastfeeding: Testosterone and its metabolites may pass into breast milk and could adversely affect a nursing infant. Use during breastfeeding is contraindicated.
Precautions & Special Populations
Cardiovascular risk: Observational data on testosterone therapy and cardiovascular outcomes are mixed. Some studies suggest a modest increase in risk for certain cardiovascular events; others show neutral or potentially beneficial effects. Patients with established cardiovascular disease, significant cardiovascular risk factors, or a history of thromboembolic events should have a thorough risk-benefit discussion with their provider before initiating therapy.
Obstructive sleep apnea: Testosterone therapy can worsen or unmask obstructive sleep apnea. Patients with known sleep apnea should be monitored, and those with risk factors should be screened before starting treatment.
Benign prostatic hyperplasia (BPH): Testosterone may cause the prostate to enlarge, potentially worsening lower urinary tract symptoms in men with existing BPH. Baseline urinary symptom assessment and ongoing monitoring are appropriate.
Fertility: Exogenous testosterone suppresses the pituitary release of luteinizing hormone (LH) and follicle-stimulating hormone (FSH), which in turn reduces intratesticular testosterone production and can significantly impair spermatogenesis. Men who wish to preserve fertility should discuss this carefully with their prescriber before starting testosterone. Fertility-sparing alternatives or adjunct therapies (such as human chorionic gonadotropin) may be appropriate in these cases.
Pediatric patients: The safety and appropriateness of testosterone therapy in patients under 18 has not been established in this context and requires specialist evaluation. Premature closure of epiphyseal growth plates is a documented risk with androgen exposure in skeletally immature patients.
Drug Interactions
Insulin and oral antidiabetic agents: Testosterone can enhance insulin sensitivity and lower blood glucose. Patients with diabetes who initiate testosterone therapy may need dose reductions of their glucose-lowering medications to avoid hypoglycemia. Blood glucose monitoring should be increased when therapy is started or doses are changed.
Oral anticoagulants (e.g., warfarin): Androgens may potentiate the anticoagulant effect of warfarin and similar medications, increasing bleeding risk. INR (international normalized ratio) should be monitored more frequently whenever testosterone doses are initiated or adjusted, and warfarin doses adjusted accordingly.
Glucocorticoids and ACTH: Concurrent use may amplify fluid retention and edema, particularly relevant for patients with cardiac or renal conditions.
5-alpha reductase inhibitors (e.g., finasteride, dutasteride): These medications block the conversion of testosterone to DHT. Their concurrent use with testosterone replacement may reduce certain androgenic effects (such as effects on hair follicles and prostate tissue) but also alters the expected therapeutic profile.
CYP3A4 inhibitors: Certain medications that inhibit the CYP3A4 enzyme pathway may increase testosterone exposure. Medication reconciliation at each visit should account for any newly added drugs with CYP3A4 activity.
Inform all healthcare providers — including dentists and emergency clinicians — that you are receiving testosterone therapy, as it can affect the interpretation of laboratory values and interact with medications used perioperatively.
Side Effects
Common: Injection site discomfort, redness, or swelling at the injection site (minimized by site rotation and proper technique); acne and oily skin; increased body and facial hair; mild fluid retention; elevated hematocrit (see monitoring section above); mood changes during peak or trough phases of the dosing cycle.
Less common but clinically significant: Worsening of benign prostatic hyperplasia; rising PSA values; worsening sleep apnea; gynecomastia (breast tissue enlargement, related to estradiol conversion); changes in cholesterol profile (HDL may decrease with long-term use).
Requiring prompt medical attention: Chest pain or shortness of breath; signs of deep vein thrombosis (leg swelling, pain, or warmth); signs of stroke; significant urinary symptoms; severe mood or behavioral changes; signs of allergic reaction including rash, facial swelling, or difficulty breathing.
Pregnancy & Breastfeeding
Testosterone cypionate is absolutely contraindicated during pregnancy. It is a known teratogen — prenatal androgen exposure can cause virilization of female fetuses and other developmental harms. If pregnancy occurs during therapy, the medication must be discontinued immediately and a healthcare provider contacted without delay. This medication is not indicated for routine use in women and should never be administered during pregnancy or breastfeeding.
Storage
Store at controlled room temperature, between 68°F and 77°F (20°C–25°C). Keep the vial in its original carton to protect from light, as light exposure can degrade the formulation over time. Do not refrigerate or freeze — the oil-based solution can crystallize or the vial may be damaged at sub-zero temperatures. If crystallization is observed, do not use the vial. Store upright and away from heat sources, direct sunlight, and areas of high humidity. Keep out of reach of children. Dispose of used vials and sharps through appropriate pharmaceutical waste and sharps disposal channels — not in household trash.
503A vs. 503B Availability
This product is available through both our 503A pharmacy (patient-specific prescriptions, compounded for named individual patients) and our 503B outsourcing facility (larger-volume preparation for licensed practitioners to administer in-office). Practitioners ordering for office stock should use the 503B channel. Individual patients with a valid prescription use the 503A pathway.

How It Works
FAQs
© 2026, TestosteroneShots.com PC
text (323)-283-9219
638 1/2 N Robertson Blvd, West Hollywood, CA 90069

The information and products provided on this website are for informational and wellness purposes only and are not intended to diagnose, treat, cure, or prevent any disease. All medical treatments are subject to evaluation and approval by a licensed healthcare provider through a telemedicine consultation. Individual results may vary, and the use of any product carries potential risks and side effects that should be discussed with a qualified medical professional. These products are not approved by the FDA unless explicitly stated, and misuse or unauthorized sharing is strictly prohibited. By using this site or purchasing any service, you agree to our Terms of Service, Telehealth Consent, and Privacy Policy. We prioritize your privacy and comply with HIPAA regulations for protected health information. Services provided by licensed physicians/nurse practitioners in states served, e.g., CA, FL, and others, via telemedicine. Check your state's telemedicine laws. Must Be 18+ or Older to Qualify For Treatment.




